DEMINERALIZATION PLANTS
WHAT IS DEMINERALIZATION?
Demineralization is a process which uses specific ion exchange resins for the removal of dissolved mineral ions present in water in form of cations such as sodium , magnesium , iron , calcium , copper etc and anions such as chlorides , sulphates, nitrates , carbonates , bicarbonates etc.
Since majority of these impurities in water are in form of dissolved salts hence De-mineralization plants produce water of high purity standards which is almost devoid of any dissolved minerals and quite similar to distilled water.
Demineralization reduces scale formation, deposition and corrosion on tubes thereby increasing life of pipes and tubes in plants and also prevents deposition of minerals in turbine blades in power plants.
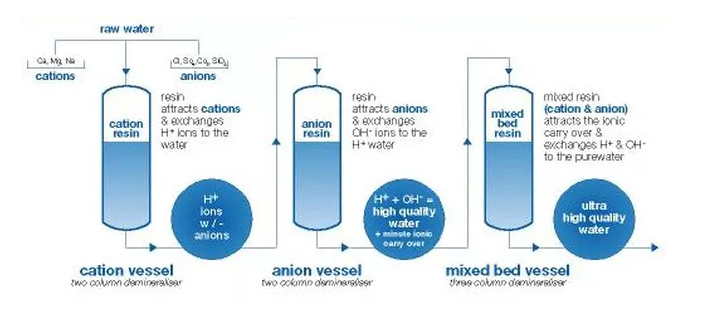
PROCESS OF DEMINERALIZATION
Ion exchange is the process in which the impurity ions present in water are replaced by ions released by the resin. The impurity ions thus get attached to the resin beads. With due passage of time , the resin beads get saturated with impurity ions and hence after sometime the need arises to re-generate or refresh the resin beads periodically.
There are basically 2 types of ion exchange resins :
1. Cation exchange resins release H+ ions or other positively charged ions in water for exchange with impurity cations present in water. These impurity cations are usually in form of metals such as sodium , magnesium , calcium , copper , iron etc.
2. Anion exchange resins release OH- or any other negatively charged ions in water for exchange with impurity anions like sulphates , chlorides , carbonates , bi-carbonates, nitrates etc.